“Prion” is a term used to describe the mysterious infectious agent responsible for several neurodegenerative diseases found in mammals. The word itself derives from ‘proteinaceous infectious particle’ and was first coined in 1982 by Stanley B. Prusiner (winner of the Noble Prize in Medicine in 1997) to refer to an infectious agent that caused Scrapie in sheep, Kuru and Creutzfeldt-Jakob Disease (CJD) in humans, and Bovine spongiform encephalopathy (BSE) in cattle. Prion diseases have always existe , but generally their incidence in the population is extremely low.
In the 1980s cows that had consumed contaminated cattle feed made from sheep carcasses infected by Scrapie, developed BSE. In the mid 1990s humans that had consumed meat from sick cows developed a type of CJD that received the name ‘mad cow disease’.
The infectious agent is a protein that is found in the membranes of normal cells (PrPc), mainly from the Central Nervous System. In these diseases, the protein adopts a misfolded confirmation (PrPSc), which is added to other neighbouring proteins, inducing a change of configuration that causes a chain reaction bringing about the generation of new infectious proteins. Consuming meat from sick cows can provoke that the normal prion protein misfolds atypically, originating the altered isoform. It is also believed that the disease was transmitted to patients who received corneal transplants and dura mater of donors patients with abnormal protein. Similarly, there were some exceptional cases of iatrogenic transmission, through the use of contaminated medical devices with organic waste from people affected, but that have not represented even 1% of the cases recorded from 19501. These diseases have very long incubation periods with very varied symptomatology over time, that lead to an extreme neurodegenerative degradation in the late stages of the disease resulting in the death of the patient.
PrPsc is very resistant to conventional sterilizing and disinfecting methods and its resistance can be increased through the use of fixing agents if the proteins have not been eliminated efficiency during the initial stages of reprocessing.
Some countries have introduced specific regulations to ensure the removal/inactivation of prion proteins in medical devices. All of them are based on the detection of patients at risk (confirmed symptomatology or those with genetic factors) and the specific care of the surgeries related to the Central Nervous System (high risk tissues): brain, spinal cord, or back of the eye. Here we show you some specific examples:
- Wherever possible, only use medical devices once.
- If this is not possible, the medical device must be retained /held in quarantine until it can be confirmed it is free from risk, or destroyed in the event of contamination being confirmed.
- Use processes that have been experimentally proven to inactivate the prion, destroying the protein.
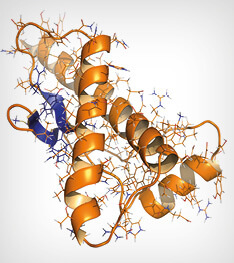
Human prion protein (hPrP), chemical structure. Associated with neurogedenerative diseases, including kuru, BSE and Creutzfeldt-Jakob. Cartoon & wireframe representation. Secondary structure coloring (blue sheets, orange helices).
The assessment of the inactivation of prions is based on in vivo experiments. The use of sodium hypochlorite at certain ppm levels, sodium hydroxide, high pH alkaline detergents, some low temperature sterilization cycles and the combined use of these alkaline detergents with low temperature cycles, have demonstrated their effectiveness. This should be demonstrated in very well defined conditions: concentration, temperature, exposure times, etc., and they are only valid for these circumstances. Steam cycles at 134 °C / 18 minutes, are highly efficient although the inactivation cannot be complete. For this, we recommend the use of a prior alkaline cleaning phase for using instruments on high risk tissues.
Some methods of sterilization, cleaning and disinfection can encourage the fixing of protein on the surfaces of the surgical instruments: alcohol, glutaraldehyde, dry heat, ethylene oxide or other aldehyde type of sterilizing agents. The countries with specific regulations on prions may advise against the use of these methods, or require a previous, non-fixing procedure before applying these technologies, for example, the use of high pH alkaline detergents where the manufacturer declares their inactivation capacity.
Finally, some countries may establish their own regulations with regard to the methods and levels of detection of residual protein on the medical devices, applying levels and additional precautions to instruments used on high-risk tissues. For example, the British recommendations HTM 0101 of 2017, establish levels below 5 µg of residual protein per risk material and lower than 8 µg for the rest. The implementation of protein detection methods can be of great assistance in the RUMED to establish risk assessment.
1 Human prion diseases: surgical lessons learned from iatrogenic prion transmission. David J. Bonda et col. Neurosurgical Focus. 2016 Jul; 41(1): E10
Other sources used:
- Guides from the WFHSS 2019: https://wfhss-guidelines.com/reusable-medical-device/
- Reducing the risk of transmission of Creutzfeldt–Jakob disease (CJD) from surgical instruments used for interventional procedures on high-risk tissues Interventional procedures guidance [IPG666]Published date: January 2020. NICE: National Institute for Health and Care Excellence
- Health Technical Memorandum (HTM) 01-01 on the management and decontamination of surgical instruments (medical devices) used in acute care. March 2013: Hygiene requirements for the reprocessing of medical devices. Recommendation of the KRINKI at the RKI and NfArM Bundesgesundheitsbl 2012 · 55:1244-1310.
- World Health Organization. Infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO Consultation, Ginebra, Suiza, 23-26 Marzo, 1999. (http://www.who.com)

Latest comments