HYDROGEN PEROXIDE & PLASMA
STERILIZER 130HPO®

130HPO® STERILIZER
Its 148-litre chamber capacity, three differentiated cycles and speed make it the optimal solution for high-volume work centres.

STERILIZING SOLUTION EXPIRY DATE
Properties of the sterilizing solution, guaranteed 30 days inside the device thanks to the exclusive integrated refrigerating system.
EFFICIENCY
- Conditioning of the load with elimination of residual humidity.
- Self-diagnosis with confirmation of the load condition suitability and consequent saving of sterilizing solution, in case of cycle cancellation.
BluKat® AUTONOMY

DESIGN

USER FRIENDLY
- EasyRUN intuitive user interface.
- Status of the unit visible from a distance, thanks to the backlit front panel.
- Easy and quick learning by users.

ERGONOMICS

PLUG & PLAY (50HPO®)

CONNECTIVITY & DOCUMENTATION
- Integrated thermal printer.
- High-capacity internal memory with track record of the last 1000 cycles.
- USB port for backing up the cycle track record.
- Ethernet port for connection to monitoring and traceability software.
Blukat® the sterilizing solution container
30 days expiry date inside the device, thanks to the refrigerated compartment that extends the life of the sterilizing solution, saving time and reducing handling.

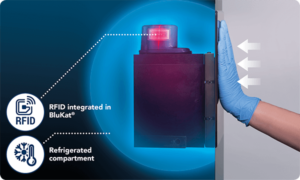
How to replace BluKat®?



